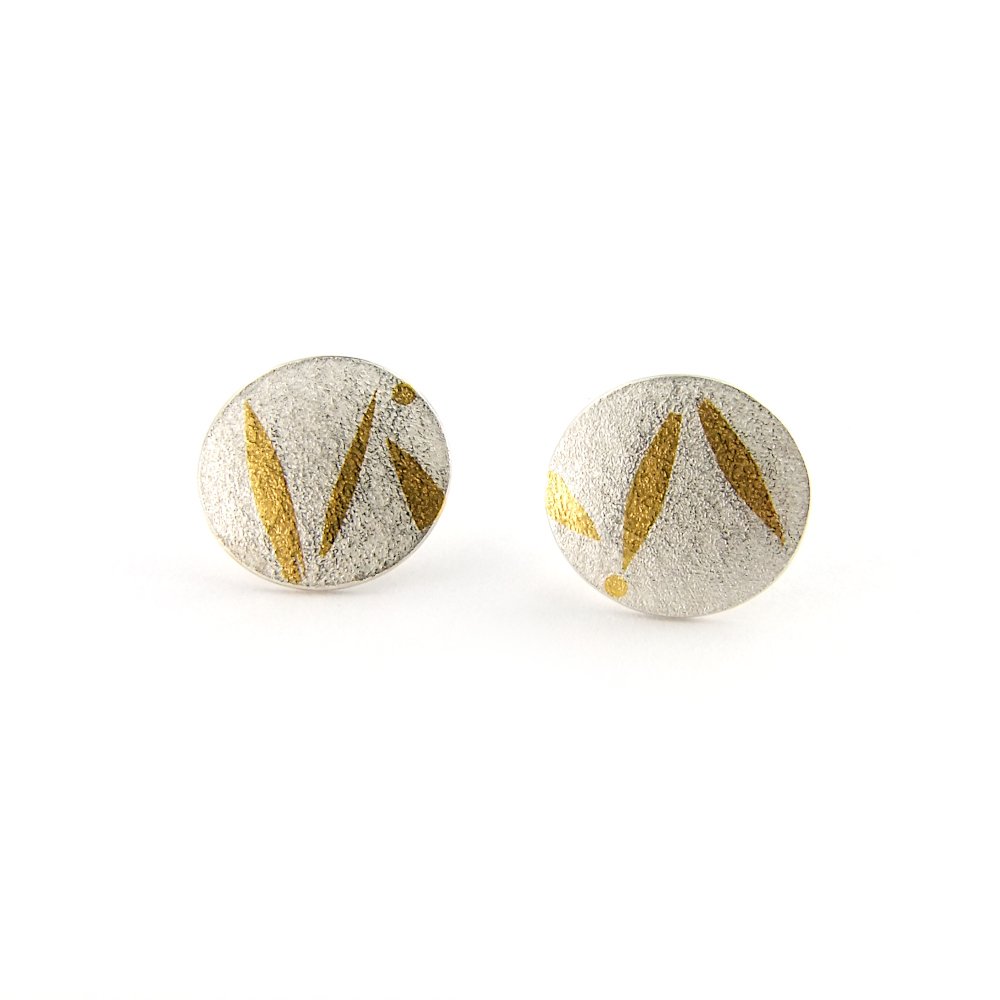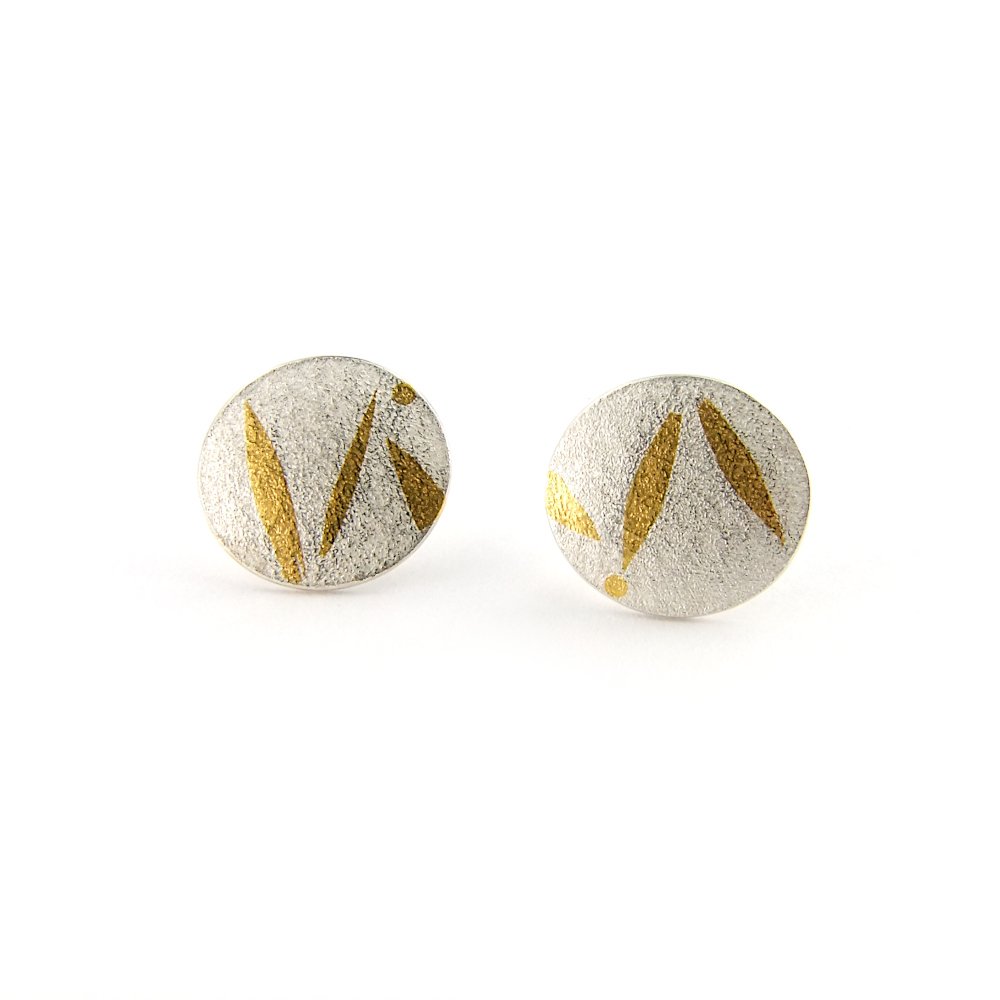In my 10th (October) Blog I wanted to write about the removal of tarnish from silver jewellery. However, October and November have been busy months and I did not manage to finish this piece.
Now, at the end of November it is almost finished but it has once more become a lengthier article and so I decided to divide it into two, more manageable pieces.
Motivation
As to my motivations for writing about this topic: When I set up my website I wrote a section for my customers (or those interested) on how to look after their jewellery. I covered tarnish removal but recently felt that it could do with updating.
More specifically, I wanted to investigate methods other than using a polishing cloth and those which worked well on textured or satin surfaces. I did some research and conducted some experiments to see which methods worked, were environmentally friendly and could be replicated at home.
What is tarnish?
Silver is a white metal which has been widely used throughout the ages. Its sought-after qualities include its high lustre, reflectivity, superior thermal and electrical conductivity as well as very good malleability and ductility.
Silver is an element with the symbol Ag, lies within Group 11 and Period 5 of the Periodic Table and has the Atomic Number 47. In its pure state silver is very soft, making it often impractical for use in jewellery. It is therefore alloyed (mixed) with copper which makes it harder and produces what we term Sterling Silver. The ratio of metals in Sterling Silver is 925 parts pure silver and 75 parts copper.
Pure silver barely reacts with oxygen, however, it reacts with sulphur-containing gasses in the air. The most common is hydrogen sulphide (for example contained in hard-boiled eggs, decomposing plants or animals.) Silver reacts with this gas and produces the compound silver sulphide (Ag2S) and it is this, we define as the brownish-black tarnish on silver. (In Sterling silver the reaction with hydrogen sulphide also produces copper sulphide (Cu2S).)
Tarnish, therefore, is the result of a chemical reaction between a metal and a gas
Early silver Athenian coin, 5th century BCE. British Museum. By I, PHGCOM, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2392720
Which metals are affected and why?
Tarnish can affect different metals. Copper, brass and aluminium can also tarnish through their exposure to oxygen or other gases, but silver needs hydrogen sulphide to form this dark layer of tarnish.
Unlike rust, however, the tarnish layer actually protects the inner layers of the metal from further tarnishing. A newly polished piece of silver, therefore, tarnishes quicker than an already tarnished piece. However, tarnish changes the original appearance of the piece and this is why most people would like it removed.
Traditional methods of removal?
There are many ways in which tarnish can be removed from silver once it has occurred, though it is best to prevent the tarnish from occurring in the first place. This can be achieved by storing the silver article in a sealed plastic bag or container, thus avoiding contact with air which may contain hydrogen sulphide.
To remove tarnish, once it has occurred, the following methods have traditionally been used to remove tarnish from silver:
by mechanical action of polishing the silver, using a silver polishing cloth
by immersing the silver into a chemical solution (silver dip)
by an electrochemical process, involving baking soda, water and aluminium
Before any tarnish is removed, the piece should be gently cleaned with warm soapy water and a soft cloth or toothbrush to remove any other dirt particles that may have accumulated.
Mechanical action – Polishing Cloths
All mechanical polishing action involves some kind of abrasive particles to remove the tarnish (as well as some silver), although some products are gentler than others. It is advisable to check on a test piece how aggressive or not your chosen product is.
Polishing cloths are soft cloths that are impregnated with a polishing compound which removes the tarnish and usually also a tarnish inhibitor which prevents (or slows down) tarnish from occurring.
These cloths are very gentle and are a good way of removing light tarnish from your silver jewellery and they work particularly well on polished surfaces. They can also be used on textured surfaces but on coarser textures or heavy tarnish they may not be able to remove all the tarnish. Using them on matte or satin surfaces will result in the surfaces being shinier than before.
Polishing cloths can also be used on pieces with 24ct gold Keum-Boo patterns. The surface will become shinier than the original finish.
Chemical solutions (silver dip)
These chemical dips are available from different companies. They work by immersing the tarnished piece of silver into the solution for a couple of minutes and then rinsing and drying it. The tarnish will have been removed.
They are not suitable for all items, especially if other materials or gemstones are included in the piece.
These dips contain an acid (in the brands I compared, these were hydrochloric acid and sulfuric acid) and other chemicals that may be harmful to the user. In the safety data sheets of two different brands I compared these substances were classed as hazardous to health.
Due to these chemicals these solutions are very difficult to safely dispose of after use.
- To be continued in Part Two -